Decades of research may ultimately determine how to target the main genetic drivers of lung cancer and other cancers, KRAS. The preliminary research results released at the annual meeting of the American Society of Clinical Oncology held in Chicago showed that the new precision cancer drug currently known as Lumakras "AMG 510" has a 50% response rate, and the follow-up data released at ASCO 2021 has expanded and confirmed these encouraging results.
The US Food and Drug Administration (FDA) has granted Sotorasib breakthrough therapy recognition, which will be marketed as Lumakras for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) patients with KRAS G12C mutations.
The results of the CodeBreakK 100 clinical study were presented at the 2020 IASLC World Lung Cancer Conference. The study evaluated Lumakras (Sotolasi) in 126 late stage NSCLC patients with KRAS G12C mutations, who had previously failed both cancer treatments with a median response rate of 37% in January 2021.
The results were updated at the 2021 ASCO annual meeting. Currently, the median follow-up time is 15.3 months, with a median overall survival of 12.5 months and a median progression free survival of 6.8 months for Lumakras. The median reaction duration was 11.1 months.

KRAS is a carcinogenic gene, which can affect the growth of lung cancer, pancreatic cancer and colorectal cancer. AMG510 is the first drug of its kind to enter clinical testing and bind to a mutated KRAS protein, thereby "turning off" the signals it sends that trigger cell division and cancer cell growth.
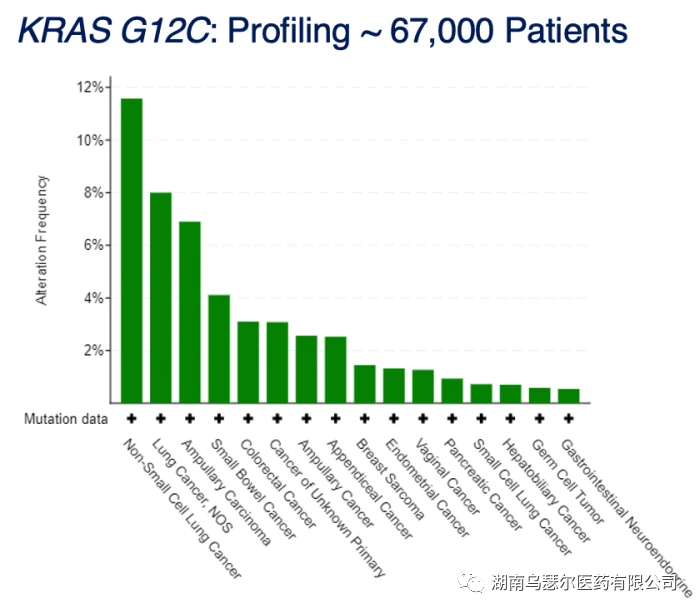
Lumakras irreversibly inhibits KRAS G12C by permanently blocking it in an inactive GDP binding state, and represents a first-class novel small molecule inhibitor that specifically binds to mutated proteins in KRAS. G12C occurs in approximately 13% of NSCLC, 3% to 5% of colorectal cancer, and 1% to 3% of other cancers.
In the CODEBREAK 100 trial, patients who simultaneously experienced TP53 (wild-type, 40%; mutant, 39%), STK11 (wild-type, 39%; mutant, 40%), and KEAP1 (wild-type) mutations also experienced reactions- Type, 44%; Mutant type, 20%) 4,5,6
More information about the KRAS oncogene
A key milestone in cancer research is the discovery of a group of genes called the RAS family. RAS is a carcinogenic gene - a gene that encodes proteins that act as switches to activate various genes to promote cell growth and division. These genes play complex roles in the normal cell cycle, responding to intracellular and extracellular signals that regulate cell growth and division rate. In addition, there are countless proteins that interact with RAS - including receptors on the cell surface - that transmit signals to cells through complex protein interaction circuits, ultimately resulting in changes in gene expression. Mutations in the RAS gene result in a permanent "on" switch, leading to uninhibited cell division and ultimately leading to cancer.
There are three types of RAS oncogenes, designated as NRAS, GRAS, and KRAS. Although mutations in all three can lead to cancer, KRAS is the most common mutated oncogene in human colorectal cancer. Approximately 40% to 50% of human colorectal cancer have mutations in the KRAS gene. Recently developed laboratory tests can distinguish tumors with this mutation from tumors with normal (also known as wild-type) KRAS.
This has several therapeutic implications. Cancer with non mutant or wild-type KRAS is sensitive to a class of biological agents called epidermal growth factor receptor (EGFR) inhibitors. EGFR is a receptor on the cell surface that binds to a growth factor called epidermal growth factor (EGF). This receptor activates the cellular pathway that promotes cell growth and division, and KRAS is one of the key participants in this process. Blocking the EGF receptor can eliminate this important signal for the sustained growth of cancer cells. The two EGFR targeted drugs studied in colon cancer are Erbitux ® (Cetuximab) and Vectibix (Panizumab).
Another exciting group of drugs specifically targeting the KRAS protein works by inhibiting an enzyme called farnesyltransferase. This enzyme is involved in activating the RAS protein. So far, these drugs cannot be used at the bedside and are currently being tested in animal models.
Other cancers also express KRAS G12C mutations