Lung cancer is one of the most common types of cancer in the United States, second only to breast cancer. In 2015 alone, 221200 new lung cancer cases were diagnosed, accounting for 13.3% of all new cancer cases. Lung cancer is the main cause of cancer deaths for men and women, accounting for 27% of all cancer deaths, and taking more lives than breast cancer, colon cancer, prostate cancer and ovarian cancer combined. From 2007 to 2011, the incidence rate and mortality of lung cancer decreased slightly.
Approximately 85% to 90% of lung cancer is non-small cell lung cancer (NSCLC), which includes several major subtypes, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Adenocarcinoma accounts for 40% of all lung cancer cases, while squamous cell carcinoma accounts for approximately 25% to 30%. Other less common types of NSCLC include carcinoid, pleomorphic, salivary adenocarcinoma, and unclassified cancer.
Like other cancers, the earlier lung cancer is discovered and treated, the better the prognosis. However, approximately 22% of lung cancer cases have already locally spread (to regional lymph nodes) at the time of diagnosis, and 57% have already metastasized to distant parts of the body. The 5-year relative survival rate of patients with regional metastasis is 27.4%, while the 5-year relative survival rate of patients with distant metastasis is only 4.2%.
The treatment of metastatic NSCLC typically includes chemotherapy, targeted therapy, immunotherapy, or a combination of these options. Most targeted therapies are small molecule compounds designed for intracellular targets or monoclonal antibodies designed for extracellular targets.
The scientific progress in cell biology and gene expression has promoted the development of new targeted therapies and changed the pattern of NSCLC treatment. Several predictive biomarkers have emerged, indicating the therapeutic efficacy of specific drugs or drug classes on specific NSCLC molecular targets. These biomarkers include allergenic epidermal growth factor receptor (EGFR) mutations (such as exon 19 deletion or exon 21, L858R mutations) and anaplastic lymphoma kinase (ALK) fusion genes.
Some clinical guidelines recommend testing for EGFR mutations and ALK gene rearrangements in specific NSCLC patients. The currently approved NSCLC therapies by the US Food and Drug Administration (FDA) include two immune checkpoint inhibitors, navumab and pembrolizumab, as well as targeted therapies such as bevacizumab, ramolizumab, afatinib, erlotinib, oxitinib, necitumumab, erlotinib, seretinib, and clotozotinib.
The continuous development of molecular analysis and targeted therapy is changing the treatment options available for non-small cell lung cancer patients. Therefore, 2015 was a landmark year for FDA approval of new therapies for non-small cell lung cancer.
Yiruisha is a new first-line therapy for EGFR mutated metastatic NSCLC
On July 13, 2015, the FDA approved gefitinib (Yiruisha; AstraZeneca), an oral tyrosine kinase inhibitor, for first-line treatment in patients with metastatic NSCLC. These patients had tumors with EGFR exon 19 deletion or exon 21 L848R substitution mutations, and received FDA approval on the same day of testing for the therascreen EGFR RGQ PCR Kit. This test can identify EGFR mutations and be used to select candidates for gefitinib treatment.
Exon 19 deletion and exon 21 L858R replacement gene mutations are the most common types of EGFR mutations. Tumors expressing these EGFR mutations are associated with tumor cell growth and metastasis in NSCLC. Gefitinib targets EGFR exon 19 deletion and exon 21 L858R mutation. Gifitinib is not suitable for patients with EGFR mutations other than exon 19 deletion or exon 21 L858R substitution mutations in tumors.
Although gefitinib was approved by the FDA for the treatment of advanced non-small cell lung cancer in 2003, due to a lack of evidence to confirm its clinical benefits, the drug voluntarily withdrew from the market in April 2012. The new FDA approval in 2015 is based on evidence of the clinical benefits of gefitinib for untreated EGFR mutated positive NSCLC patients; Gifitinib has been designated as an orphan drug for this indication.
Richard Pazdur, MD, Director of the FDA Hematology and Oncology Products Office, said, "Iresa provides another effective first-line treatment option for selected lung cancer patients. This approval provides further support for highly targeted cancer treatment methods
Mechanism of action
Gifitinib is a tyrosine kinase inhibitor that blocks proteins involved in cancer cell proliferation with EGFR exon 19 deletion and exon 21 L858R substitution mutations - two EGFR activated mutations contribute to tumor cell growth, angiogenesis factors, and metastasis.
Administration and administration
The recommended dose of gefitinib is 250 milligrams, once a day, taken orally with or without food until the disease progresses or until unacceptable toxicity occurs. If one dose is missed, it should not be taken within 12 hours after the missed dose.
Gifitinib can be administered orally as a 250 milligram tablet.
Clinical Research IFUM Clinical Trials
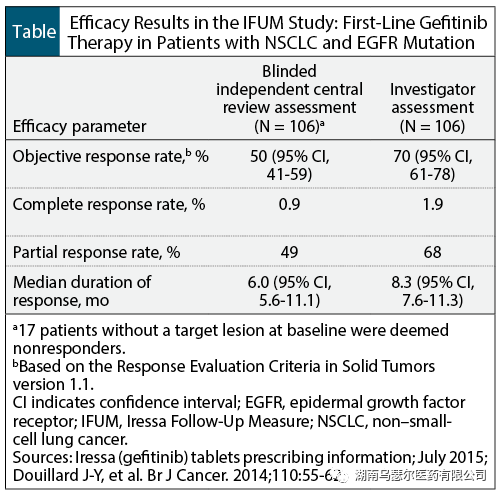
FDA approval of gefitinib is based on data from IRESSA Follow-up Measures (IFUM) clinical trials, a multicenter, single arm, open-label study consisting of 106 untreated patients with metastatic NSCLC containing EGFR (median age 65 years) with exon 19 deletion or exon 21 L858R replacement mutation. The median duration of treatment with gefitinib was 8 months.
The primary efficacy endpoint is the objective response rate. Secondary endpoints include response duration, progression free survival, overall survival, and safety and tolerability.
According to the evaluation of the researchers, gefitinib treatment showed an objective response rate of 70%, as well as a 50% objective response rate based on blind independent central review (Table). The remission rate of patients with EGFR exon 19 deletion mutation is similar to that of patients with exon 21 L858R substitution mutation.
IPASS study: NSCLC plus EGFR mutation
The results of the IFUM clinical trial were supported by exploratory analysis of patient subgroups in the Irisa Pan Asia Study (IPASS). IPASS is a randomized, multicenter, open-label study conducted on 186 EGFR mutation positive NSCLC patients. Patients were randomly assigned to receive 250 mg of gefitinib or carboplatin plus paclitaxel.
The median progression free survival of patients receiving gefitinib treatment was 10.9 months, compared to 7.4 months in the carboplatin paclitaxel treatment group (hazard ratio, 0.54; 95% confidence interval [CI], 0.38-0.79). The objective response rate of gefitinib was 67% (95% CI, 56-77), while the objective response rate of carboplatin plus paclitaxel was 41% (95% CI, 31-51). The median remission duration of gefitinib was 9.6 months, while that of carboplatin plus paclitaxel was 5.5 months.
adverse event
The adverse reactions of gefitinib treatment were evaluated in a randomized, double-blind, placebo-controlled clinical trial involving patients receiving 250 milligrams of gefitinib daily with a median duration of 2.9 months. The most common (≥ 10%) adverse reactions associated with gefitinib treatment at all levels (and greater than placebo) include skin reactions (47%), diarrhea (29%), and vomiting (14%).
The most common grade 3 or 4 adverse reactions reported by gefitinib (≥ 2% and more than placebo) were diarrhea (3%), decreased appetite (2.3%), and skin reactions (2%).
Approximately 5% of patients receiving gefitinib treatment stop treatment due to adverse reactions. The most common adverse reactions leading to discontinuation of gefitinib are nausea (0.5%), vomiting (0.5%), and diarrhea (0.4%).
Drug interactions
Cytochrome (CY) P3A4 inducer. For patients taking the potent CYP3A4 inducer, the dosage of gefitinib should be increased to 500mg per day; After discontinuing the potent CYP3A4 inducer for 7 days, a dose of 250 milligrams can be restored.
CYP3A4 inhibitor. Adverse reactions should be monitored in patients who take both potent CYP3A4 inhibitors and gefitinib simultaneously.
Drugs that affect gastric acidity and alkalinity. If possible, gefitinib should not be used together with proton pump inhibitors.
Patients taking warfarin experience bleeding. Patients who take both warfarin and gefitinib should be monitored for changes in prothrombin time or internationally standardized ratios.
Warnings and precautions
Interstitial lung disease. There have been reports of treatment with gefitinib for interstitial lung disease. If respiratory symptoms worsen, gefitinib should be discontinued. If diagnosed with interstitial lung disease, gefitinib should be discontinued.
Hepatotoxicity. Patients taking gefitinib should undergo regular liver function tests. Patients with liver function abnormalities ≥ level 2 should discontinue gefitinib. Patients with severe liver function damage should discontinue gefitinib.
Gastrointestinal perforation. If gastrointestinal perforation occurs, gefitinib should be permanently discontinued.
Diarrhea. For ≥ grade 3 diarrhea, gefitinib should be discontinued.
Eye diseases. If the patient exhibits severe or worsening symptoms of eye diseases, including keratitis, gefitinib should be discontinued. If persistent ulcerative keratitis occurs, gefitinib should be discontinued.
Bullous and exfoliative skin diseases. Patients with severe blisters, blisters, or peeling symptoms (including erythema multiforme and bullous dermatitis) should discontinue or discontinue gefitinib treatment.
Embryo fetal toxicity. Taking gefitinib to pregnant women may cause harm to the fetus.
Used in specific populations
Pregnancy. Taking gefitinib to pregnant women may cause harm to the fetus. Women with reproductive potential should be advised to take contraceptive measures during and at least 2 weeks after completion of gefitinib treatment.
Lactation period. It has not been determined whether gefitinib will be excreted from human milk. Women should be advised to stop breastfeeding during treatment with gefitinib.
Pediatric use. The safety and efficacy of gefitinib in pediatric patients have not been determined yet.
Used by the elderly. No overall difference in the safety of gefitinib was observed between patients aged>65 years and younger patients. 12 data is insufficient to determine whether the efficacy of gefitinib differs between these two age groups.
Renal insufficiency. Clinical trials have not been conducted in patients with severe renal dysfunction.
Liver function damage. Patients with moderate or severe liver function impairment receiving gefitinib treatment should monitor adverse reactions.
conclusion
FDA approved oral tyrosine kinase inhibitor gefitinib as an important targeted treatment option for NSCLC patients carrying EGFR mutations. Among this group of EGFR mutated patients, gefitinib showed important and significant clinical benefits, including a 70% objective response rate, a median response duration of 8.3 months, a 50% objective response rate, and a median response duration of 6 months.